2.2 Water
Learning Objectives
By the end of this section, you will be able to:
- Describe the properties of water that are critical to maintaining life
- Explain why water is an excellent solvent
- Provide examples of water’s cohesive and adhesive properties
- Discuss the role of acids, bases, and buffers in homeostasis
Watch a video about why we need oxygen and how it causes problems for living things.
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60–70 percent of your body is made up of water. Without it, life simply would not exist.
The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the processes of life. Life originally evolved in a watery environment, and most of an organism’s cellular chemistry and metabolism occur inside the watery contents of the cell’s cytoplasm. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions that leads to generating acids and bases. Understanding these characteristics of water helps to elucidate its importance in maintaining life.
Water Is Polar
The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no net charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Oxygen is more electronegative than hydrogen, making it more likely that a shared electron would be near the oxygen nucleus than the hydrogen nucleus, thus generating the partial negative charge near the oxygen (Figure 2.13).

Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds (Figure 2.13). When a substance readily forms hydrogen bonds with water, it can dissolve in water and is referred to as hydrophilic (“water-loving”). Hydrogen bonds are not readily formed with nonpolar substances like oils and fats . These nonpolar compounds are hydrophobic (“water-fearing”) and will not dissolve in water (Figure 2.14).

Water’s States: Gas, Liquid, and Solid
The formation of hydrogen bonds is an important quality of the liquid water that is crucial to life as we know it. As water molecules make hydrogen bonds with each other, water takes on some unique chemical characteristics compared to other liquids and, since living things have a high water content, understanding these chemical features is key to understanding life. In liquid water, hydrogen bonds constantly form and break as the water molecules slide past each other. The water molecules’ motion (kinetic energy) causes the bonds to break due to the heat contained in the system. When the heat rises as water boils, the water molecules’ higher kinetic energy causes the hydrogen bonds to break completely and allows water molecules to escape into the air as gas (steam or water vapour). Alternatively, when water temperature reduces and water freezes, the water molecules form a crystalline structure maintained by hydrogen bonding (there is not enough energy to break the hydrogen bonds) that makes ice less dense than liquid water, a phenomenon that we do not see when other liquids solidify.
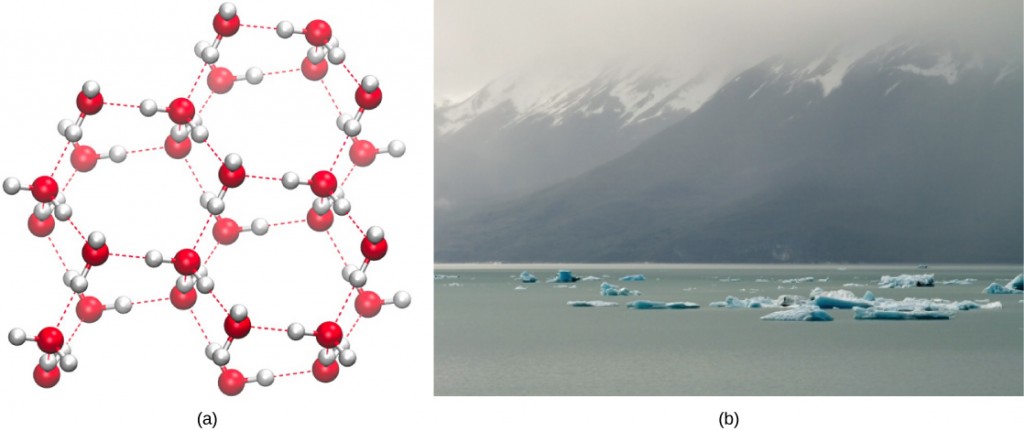
Water’s lower density in its solid form is due to the way hydrogen bonds orient as they freeze (Figure 2.15a): the water molecules push farther apart compared to liquid water. With most other liquids, solidification when the temperature drops includes lowering kinetic energy between molecules, allowing them to pack even more tightly than in liquid form and giving the solid a greater density than the liquid. The expansion of ice relative to liquid water causes the detrimental effect of freezing on living organisms. The ice crystals that form upon freezing rupture the delicate membranes essential for living cells to function, irreversibly damaging them. Cells can only survive freezing if another liquid like glycerol temporarily replaces the water in them.
The lower density of ice, as Figure 2.15b depicts, an anomaly causes it to float at the surface of liquid water, such as in an iceberg or ice cubes in a glass of water. In lakes and ponds, ice will form on the water’s surface creating an insulating barrier that protects the animals and plant life in the pond from freezing. Without this insulating ice layer, plants and animals living in the pond would freeze in the solid block of ice and could not survive.
Water’s High Heat Capacity Stabilizes Temperature
Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water can absorb or release large amounts of energy and the temperature changes only minimally. Water has the highest specific heat capacity of any liquids. We define specific heat capacity as the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius. For water, this amount is one calorie. It therefore takes water a long time to heat and a long time to cool. In fact, water’s specific heat capacity is about five times more than that of sand. This explains why the land cools faster than the sea.
Water moderates temperature changes within organisms and in their environments. Warm blooded animals use water to more evenly disperse heat in their bodies: it acts in a similar manner to a car’s cooling system, transporting heat from warm places to cool places, causing the body to maintain a more even temperature. As energy input continues, the balance between hydrogen bond formation and destruction swings toward the destruction side. More bonds are broken than are formed. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body.
Water Is an Excellent Solvent
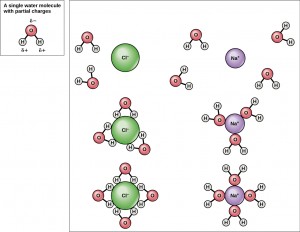
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent—a substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt (NaCl) mixed in water, the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions (Figure 2.16). A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Water Is Cohesive
Have you ever filled up a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water actually forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. When you drop a small scrap of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the water molecules. Cohesion and surface tension keep the water molecules intact and the item floating on the top. It is even possible to “float” a steel needle on top of a glass of water if you place it gently, without breaking the surface tension (Figure 2.17).

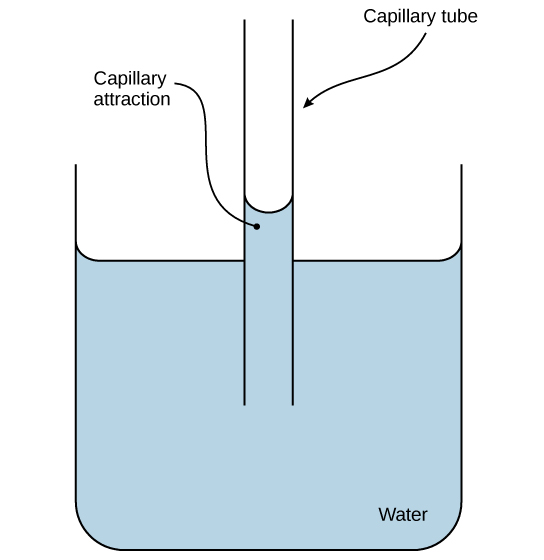
These cohesive forces are also related to the water’s property of adhesion, or the attraction between water molecules and other molecules. This is observed when water “climbs” up a straw placed in a glass of water. You will notice that the water appears to be higher on the sides of the straw than in the middle. This is because the water molecules are attracted to the straw and therefore adhere to it. We call this type of adhesion capillary action, as Figure 2.18 illustrates.
Why are cohesive and adhesive forces important for life? Cohesive and adhesive forces are important for transporting water from the roots to the leaves in plants. These forces create a “pull” on the water column. This pull results from the tendency of water molecules evaporating on the plant’s surface to stay connected to water molecules below them, and so they are pulled along. Plants use this natural phenomenon to help transport water from their roots to their leaves. Without these properties of water, plants would be unable to receive the water and the dissolved minerals they require. In another example, insects such as the water strider, as Figure 2.19 shows, use the water’s surface tension to stay afloat on the water’s surface layer and even mate there.

CONCEPT IN ACTION
To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! Website.
Acids, Bases, pH, and Buffers
Water dissociates, although sparingly, to produce H+ and OH– according to the equation
H2O(l) ⇌ H+(aq) + OH−(aq)
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base (alkalinity) exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.20).
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0 at 25°C. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).

Watch this video called MedicCastExtra – pH Scale for a straightforward explanation of pH and its logarithmic scale.
Acids are substances that provide hydrogen ions (H+) and lower pH, whereas bases accept hydrogen ions or provide hydroxide ions (OH–) and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Conversely, bases are those substances that readily donate OH–. The OH– ions combine with H+ to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH– rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will denature. Deviation outside of the pH range can induce coma or even cause death.
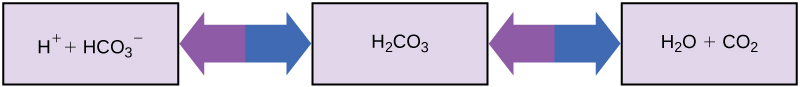
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range (Figure 2.21). This buffer system involves carbonic acid (H2CO3) and bicarbonate (HCO3–) anion. If too much H+ enters the body, bicarbonate will combine with the H+ to create carbonic acid and limit the decrease in pH. Likewise, if too much OH– is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. The H+ ions can combine with the OH– ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Other examples of buffers are antacids that some people use to combat excess stomach acid. Many of these over-the-counter medications work in the same way as blood buffers, usually with at least one ion capable of absorbing hydrogen and moderating pH, bringing relief to those who suffer “heartburn” after eating. Water’s unique properties that contribute to this capacity to balance pH—as well as water’s other characteristics—are essential to sustaining life on Earth.
Section Summary
Water has many properties that are critical to maintaining life. It is polar, allowing for the formation of hydrogen bonds, which allow ions and other polar molecules to dissolve in water. Therefore, water is an excellent solvent. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. Water’s cohesive forces allow for the property of surface tension. All of these unique properties of water are important in the chemistry of living organisms.
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high concentration of hydrogen ions is acidic and has a low pH value. A solution with a high concentration of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7.0 being neutral at 25°C. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.
Exercises
Glossary
acid: a substance that donates hydrogen ions and therefore lowers pH
adhesion: the attraction between water molecules and molecules of a different substance
base: a substance that absorbs hydrogen ions or produces hydroxide ions and therefore raises pH
buffer: a solution that resists a change in pH by absorbing or releasing hydrogen or hydroxide ions
calorie: amount of heat required to change the temperature of one gram of water by one degree Celsius
capillary action: occurs because water molecules are attracted to charges on the inner surfaces of narrow tubular structures such as glass tubes, drawing the water molecules to the tubes’ sides
cohesion: the intermolecular forces between water molecules caused by the polar nature of water; creates surface tension
dissociation: release of an ion from a molecule such that the original molecule now consists of an ion and the charged remains of the original, such as when water dissociates into H+ and OH–
evaporation: the release of water molecules from liquid water to form water vapour
heat of vaporization of water: the amount of energy required for liquid water to turn into water vapour
hydrophilic: describes a substance that dissolves in water; water-loving
hydrophobic: describes a substance that does not dissolve in water; water-fearing
litmus paper: filter paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator
pH scale: a scale ranging from 0 to 14 that measures the approximate concentration of hydrogen ions of a substance
solvent: a substance capable of dissolving another substance
specific heat capacity: the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius
sphere of hydration: when a polar water molecule surrounds charged or polar molecules thus keeping them dissolved and in solution
surface tension: the cohesive force at the surface of a body of liquid that prevents the molecules from separating
temperature: a measure of molecular motion
References
Humphrey, W., Dalke, A. and Schulten, K., “VMD—Visual Molecular Dynamics”, J. Molec. Graphics, 1996, vol. 14, pp. 33-38. http://www.ks.uiuc.edu/Research/vmd/
Media Attributions
- Figure 2.13 by Rao, A., Fletcher, S., Ryan, K., Tag, A. and Hawkins, A. Department of Biology, Texas A&M University
- Figure 2.14 by Gautam Dogra
- Figure 2.15
- (a) ice lattice by Jane Whitney
- (b) by Carlos Ponte
- Figure 2.17 by Cory Zanker
- Figure 2.18 modification of work by Pearson-Scott Foresman, donated to the Wikimedia Foundation
- Figure 2.19 by Tim Vickers
- Figure 2.20 modification of work by Edward Stevens


