5.2 Metabolic Pathways
Learning Objectives
By the end of this section, you will be able to:
- Explain metabolic pathways and describe the two major types
- Discuss how chemical reactions play a role in energy transfer
Watch a video about heterotrophs.
Scientists use the term bioenergetics to discuss the concept of energy flow (Figure 5.5) through living systems, such as cells. Cellular processes such as building and breaking down complex molecules occur through stepwise chemical reactions. Some of these chemical reactions are spontaneous and release energy; whereas, others require energy to proceed. Just as living things must continually consume food to replenish what they have used, cells must continually obtain more energy to replenish that which the many energy-requiring chemical reactions that constantly take place use. All of the chemical reactions that transpire inside cells, including those that use and release energy, are the cell’s metabolism.
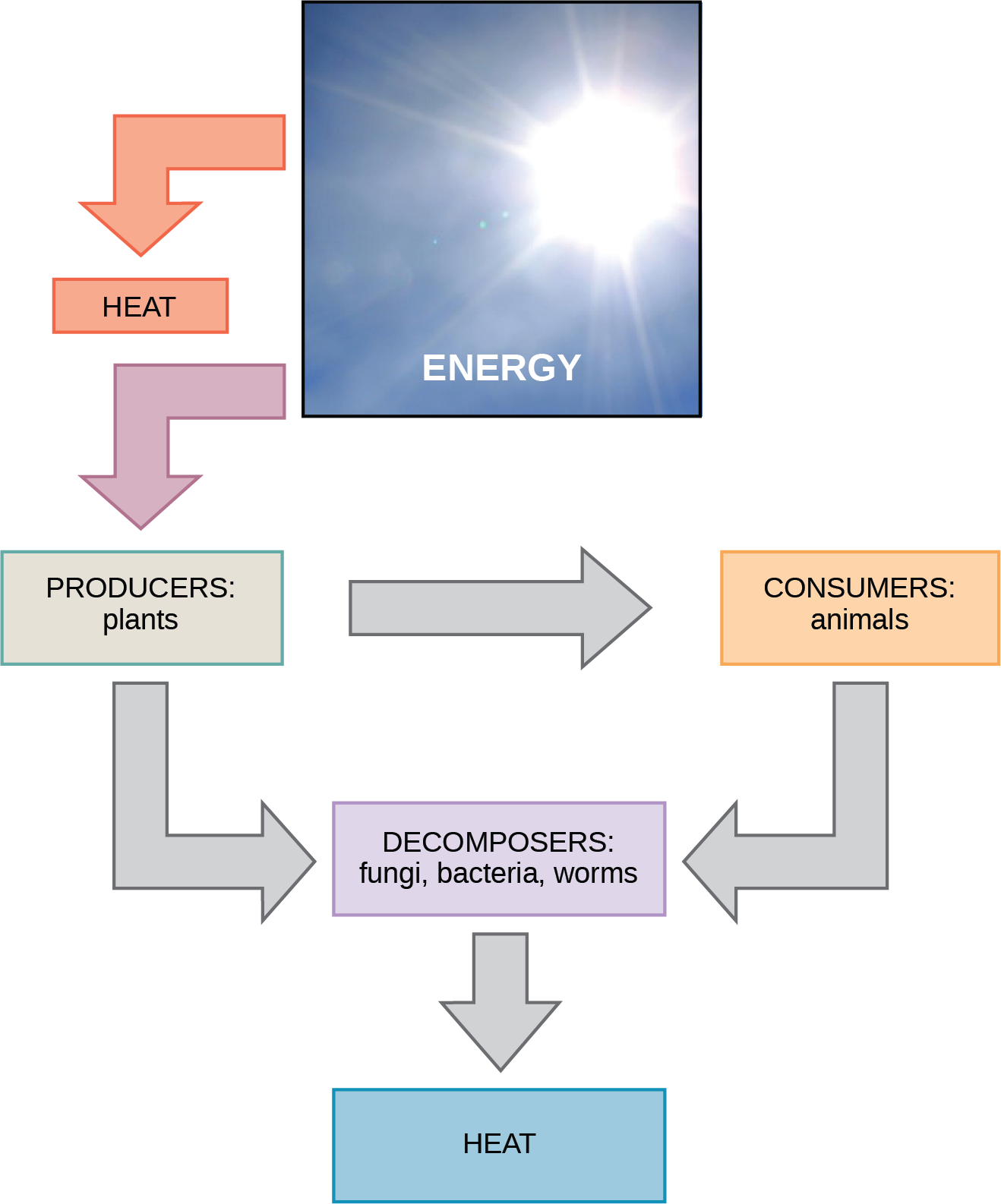
Carbohydrate Metabolism
Sugar (a simple carbohydrate) metabolism (chemical reactions) is a classic example of the many cellular processes that use and produce energy. Living things consume sugar as a major energy source, because sugar molecules have considerable energy stored within their bonds. The following equation describes the breakdown of glucose (C6H12O6), a simple sugar:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + energy
Consumed carbohydrates have their origins in photosynthesizing organisms like plants (Figure 5.6). During photosynthesis, plants use the energy of sunlight to convert carbon dioxide gas (CO2) into sugar molecules, like glucose. Because this process involves synthesizing a larger, energy-storing molecule, it requires an energy input to proceed. The following equation (notice that it is the reverse of the previous equation) describes the synthesis of glucose:
6 CO2 + 6 H2O + energy → C6H12O6 + 6 O2
During photosynthesis chemical reactions, energy is in the form of a very high-energy molecule scientists call ATP. This is the primary energy currency of all cells. Just as the dollar is the currency we use to buy goods, cells use ATP molecules as energy currency to perform immediate work. The sugar (glucose) is stored as starch or glycogen. Energy-storing polymers like these break down into glucose to supply ATP molecules.

Solar energy is required to synthesize a glucose molecule during the photosynthesis reactions. In photosynthesis, light energy from the sun initially transforms into chemical energy that temporally stores itself in the energy carrier molecules ATP and NADPH (reduced nicotinamide adenine dinucleotide phosphate). Photosynthesis later uses the stored energy in ATP and NADPH to build one glucose molecule from six molecules of CO2. This process is analogous to eating breakfast in the morning to acquire energy for your body that you can use later in the day. Under ideal conditions, energy from 18 molecules of ATP is required to synthesize one glucose molecule during photosynthesis reactions. Glucose molecules can also combine with and convert into other sugar types. When an organism consumes sugars, glucose molecules eventually make their way into each organism’s living cell. Inside the cell, each sugar molecule breaks down through a complex series of chemical reactions. The goal of these reactions is to harvest the energy stored inside the sugar molecules. The harvested energy makes high-energy ATP molecules, which perform work, powering many chemical reactions in the cell. The amount of energy needed to make one glucose molecule from six carbon dioxide molecules is 18 ATP molecules and 12 NADPH molecules (each one of which is energetically equivalent to three ATP molecules), or a total of 54 molecule equivalents required for synthesizing one glucose molecule. This process is a fundamental and efficient way for cells to generate the molecular energy that they require.
Metabolic Pathways
The processes of making and breaking down sugar molecules illustrate two types of metabolic pathways. A metabolic pathway is a series of interconnected biochemical reactions that convert a substrate molecule or molecules, step-by-step, through a series of metabolic intermediates, eventually yielding a final product or products. In the case of sugar metabolism, the first metabolic pathway synthesized sugar from smaller molecules, and the other pathway broke sugar down into smaller molecules. Scientists call these two opposite processes—the first requiring energy and the second producing energy—anabolic (building) and catabolic (breaking down) pathways, respectively. Consequently, building (anabolism) and degradation (catabolism) comprise metabolism.
EVOLUTION CONNECTION
Evolution of Metabolic Pathways
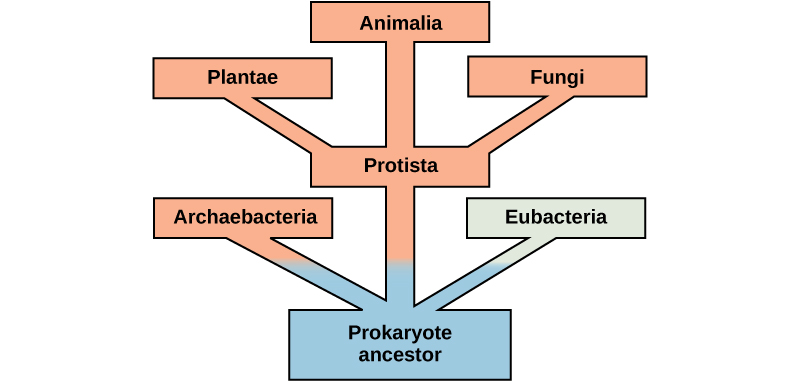
There is more to the complexity of metabolism than understanding the metabolic pathways alone. Metabolic complexity varies from organism to organism. Photosynthesis is the primary pathway in which photosynthetic organisms like plants (planktonic algae perform the majority of global photosynthesis) harvest the sun’s energy and convert it into carbohydrates. The by-product of photosynthesis is oxygen, which some cells require to carry out cellular respiration. During cellular respiration, oxygen aids in the catabolic breakdown of carbon compounds, like carbohydrates. Among the products are CO2 and ATP. In addition, some eukaryotes perform catabolic processes without oxygen (fermentation); that is, they perform or use anaerobic metabolism.
Organisms probably evolved anaerobic metabolism to survive (living organisms came into existence about 3.8 billion years ago, when the atmosphere lacked oxygen). Despite the differences between organisms and the complexity of metabolism, researchers have found that all branches of life share some of the same metabolic pathways, suggesting that all organisms evolved from the same ancient common ancestor (Figure 5.7). Evidence indicates that over time, the pathways diverged, adding specialized enzymes to allow organisms to better adapt to their environment, thus increasing their chance to survive. However, the underlying principle remains that all organisms must harvest energy from their environment and convert it to ATP to carry out cellular functions.
Anabolic and Catabolic Pathways
Anabolic pathways require an input of energy to synthesize complex molecules from simpler ones. Synthesizing sugar from CO2 is one example. Other examples are synthesizing large proteins from amino acid building blocks, and synthesizing new DNA strands from nucleic acid building blocks. These biosynthetic processes are critical to the cell’s life, take place constantly, and demand energy that ATP and other high-energy molecules like NADH (reduced nicotinamide adenine dinucleotide) and NADPH provide.
ATP is an important molecule for cells to have in sufficient supply at all times. The breakdown of sugars illustrates how a single glucose molecule can store enough energy to make a great deal of ATP, 36 to 38 molecules. This is a catabolic pathway. Catabolic pathways involve degrading (or breaking down) complex molecules into simpler ones. Molecular energy stored in complex molecule bonds release in catabolic pathways and harvest in such a way that it can produce ATP. Other energy-storing molecules, such as fats, also break down through similar catabolic reactions to release energy and make ATP (Figure 5.8).
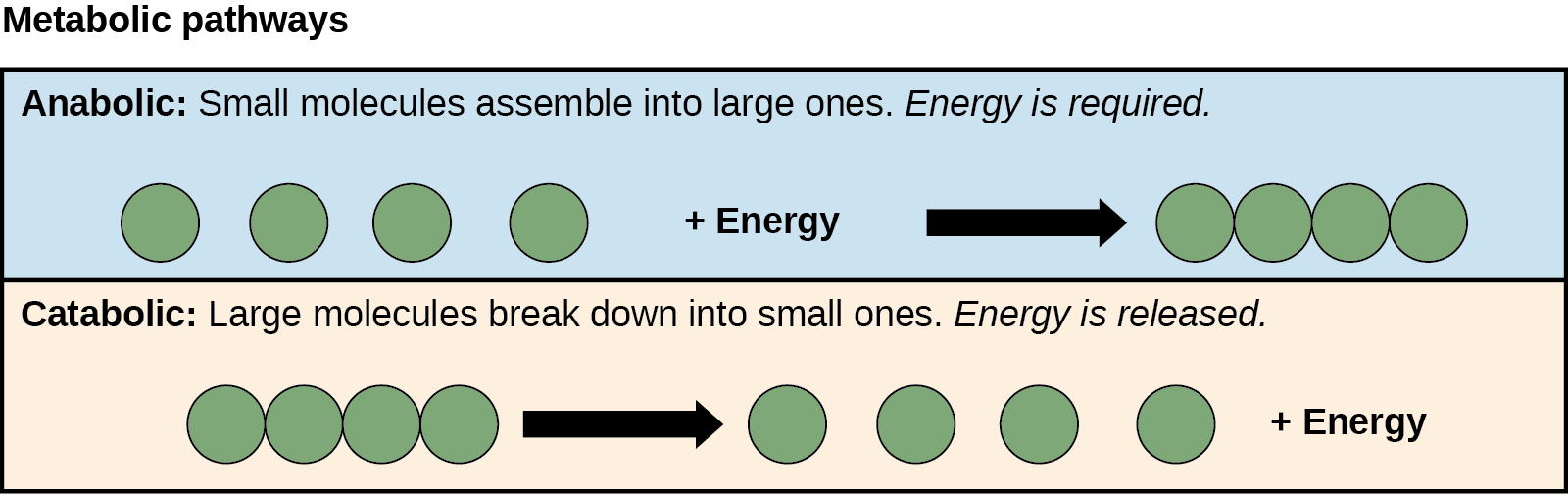
It is important to know that metabolic pathway chemical reactions do not take place spontaneously. A protein called an enzyme facilitates or catalyzes each reaction step. Enzymes are important for catalyzing all types of biological reactions—those that require energy as well as those that release energy.
Electrons and Energy
Energy production within a cell involves many coordinated chemical pathways. Most of these pathways are combinations of oxidation and reduction reactions. Oxidation and reduction occur in tandem. An oxidation reaction strips an electron from an atom in a compound, and the addition of this electron to another compound is a reduction reaction. Because oxidation and reduction occur together, these pairs of reactions are called oxidation-reduction reactions, or redox reactions.
The removal of an electron from a molecule, oxidizing it, results in a decrease in potential energy in the oxidized compound. The electron (sometimes as part of a hydrogen atom) does not remain unbonded, however, in the cytoplasm of a cell. Rather, the electron is shifted to a second compound, reducing the second compound. The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced compound). The transfer of electrons between molecules is important because most of the energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the form of electrons allows the cell to transfer and use energy in an incremental fashion—in small packages rather than in a single, destructive burst. This Unit focuses on the extraction of energy from food. You will see that as you track the path of the transfers, you are tracking the path of electrons moving through metabolic pathways.
Electron Carriers
In living systems, a small class of compounds functions as electron shuttles: they bind and carry high-energy electrons between compounds in pathways. The principal electron carriers we will consider are derived from the B vitamin group and are derivatives of nucleotides. These compounds can be easily reduced (that is, they accept electrons) or oxidized (they lose electrons). Nicotinamide adenine dinucleotide (NAD, Figure 5.9) is derived from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron).
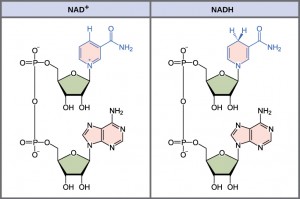
NAD+ can accept electrons from an organic molecule according to the general equation:
RH (Reducing Agent) + NAD+ (Oxidizing Agent) ⟶ R (Oxidized) + NADH (Reduced)
When electrons are added to a compound, the compound is reduced. A compound that reduces another is called a reducing agent. In the above equation, RH is a reducing agent, and NAD+ is reduced to NADH. When electrons are removed from compound, the compound is oxidized. A compound that oxidizes another is called an oxidizing agent. In the above equation, NAD+ is an oxidizing agent, and RH is oxidized to R.
Similarly, flavin adenine dinucleotide (FAD) is derived from vitamin B2, also called riboflavin. Its reduced form is FADH2. A second variation of NAD, NADP, contains an extra phosphate group. Both NAD+ and FAD are extensively used in energy extraction from sugars, and NADP plays an important role in anabolic reactions and photosynthesis.
Section Summary
Cells perform the functions of life through various chemical reactions. A cell’s metabolism refers to the combination of chemical reactions that take place within it. Catabolic reactions break down complex chemicals into simpler ones and are associated with energy release. Anabolic processes build complex molecules out of simpler ones and require energy.
Exercises
Glossary
anabolism: building up larger molecules from smaller molecules
catabolism: breaking down larger molecules into smaller molecules
oxidation: loss of electron(s) by a chemical species
reduction: gain of electron(s) by a chemical species
Media Attribution
- Figure 5.6 “acorn”: modification of work by Noel Reynolds; “squirrel”: modification of work by Dawn Huczek

