2.3 Carbon
Learning Objectives
By the end of this section, you will be able to:
- Explain why carbon is important for life
- Describe the role of functional groups in biological molecules
Cells contain many complex molecules called macromolecules, such as proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas except carbon dioxide) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of six (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
Hydrocarbons
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4) described above. We often use hydrocarbons in our daily lives as fuels—like the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which releases when these molecules burn (oxidize). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms, as Figure 2.22 illustrates. The shape of its electron orbitals determines the shape of the methane molecule’s geometry, where the atoms reside in three dimensions. The carbons and the four hydrogen atoms form a tetrahedron, with four triangular faces. For this reason, we describe methane as having tetrahedral geometry.
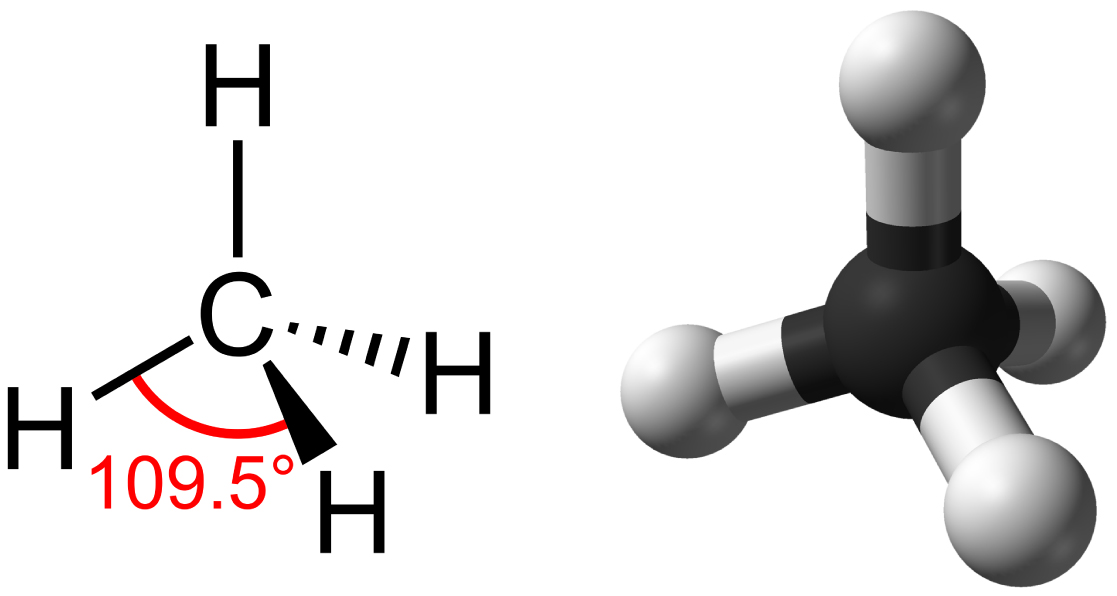
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds, and each type of bond affects the molecule’s geometry in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Hydrocarbon Chains
Successive bonds between carbon atoms form hydrocarbon chains. These may be branched or unbranched. Furthermore, a molecule’s different geometries of single, double, and triple covalent bonds alter the overall molecule’s geometry as Figure 2.23 illustrates. The hydrocarbons ethane, ethene (also called ethylene), and ethyne serve as examples of how different carbon-to-carbon bonds affect the molecule’s geometry. The names of all three molecules start with the prefix “eth-,” which is the prefix for two-carbon backbone. The suffixes “-ane,” “-ene,” and “-yne” refer to the presence of single, double, or triple carbon-carbon bond, respectively. Thus, propane, propene, and propyne follow the same pattern with three carbon molecules, butane, butene, and butyne for four carbon molecules, and so on. Double and triple bonds change the molecule’s geometry: single bonds allow rotation along the bond’s axis; whereas, double bonds lead to a planar configuration and triple bonds to a linear one. These geometries have a significant impact on the shape a particular molecule can assume.

Hydrocarbon Rings
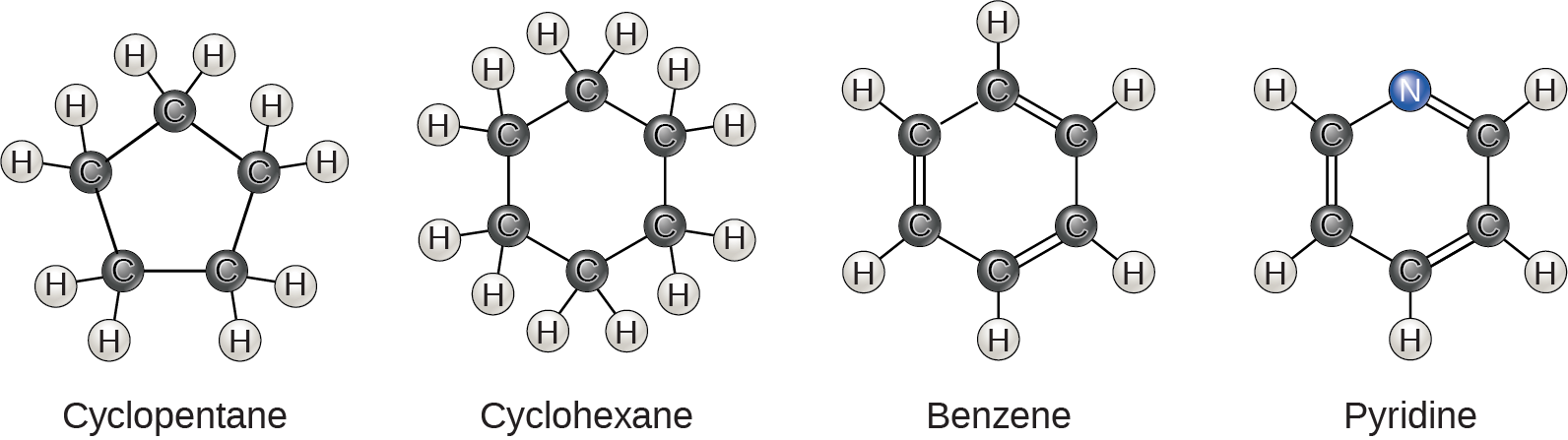
So far, the hydrocarbons we have discussed have been aliphatic hydrocarbons, which consist of linear chains of carbon atoms. Another type of hydrocarbon, aromatic hydrocarbons, consists of closed rings of carbon atoms with alternating single and double bonds. We find ring structures in aliphatic hydrocarbons, sometimes with the presence of double bonds, which we can see by comparing cyclohexane’s structure to benzene in Figure 2.24. Examples of biological molecules that incorporate the benzene ring include some amino acids and cholesterol and its derivatives, including the hormones estrogen and testosterone. We also find the benzene ring in the herbicide 2,4-D. Benzene is a natural component of crude oil and has been classified as a carcinogen. Some hydrocarbons have both aliphatic and aromatic portions. Beta-carotene is an example of such a hydrocarbon.
Isomers
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Isomers are molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds. Structural isomers (like butane and isobutane in Figure 2.25a) differ in the placement of their covalent bonds: both molecules have four carbons and ten hydrogens (C4H10), but the different atom arrangement within the molecules leads to differences in their chemical properties. For example, butane is suited for use as a fuel for cigarette lighters and torches; whereas, isobutane is suited for use as a refrigerant and a propellant in spray cans.
Geometric isomers, alternatively have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms, especially in carbon-to-carbon double bonds. In the simple molecule butene (C4H8), the two methyl groups (-CH3) can be on either side of the double covalent bond central to the molecule, as Figure 2.25b illustrates. When the carbons are bound on the same side of the double bond, this is the cis configuration. If they are on opposite sides of the double bond, it is a trans configuration. In the trans configuration, the carbon atoms forms a more or less linear structure; whereas, the carbon atoms in the cis configuration makes a bend (change in direction) in the carbon backbone.
VISUAL CONNECTION

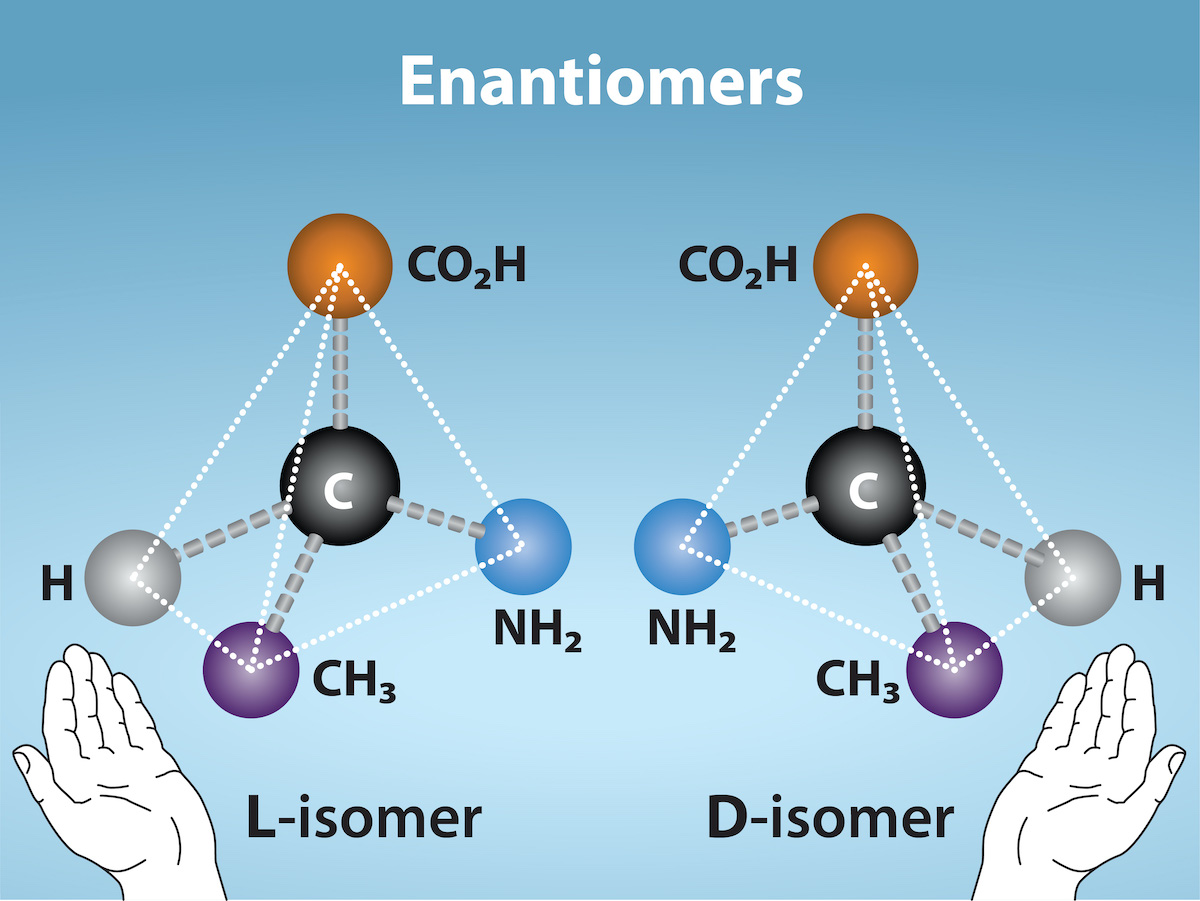
Enantiomers
Enantiomers are molecules that share the same chemical structure and chemical bonds but differ in the three-dimensional placement of atoms so that they are non-superimposable mirror images (Figure 2.25c). Figure 2.26 shows an amino acid alanine example, where the two structures are non-superimposable. In nature, the L-forms of amino acids are predominant in proteins. Some D forms of amino acids are seen in the cell walls of bacteria and polypeptides in other organisms. Similarly, the D-form of glucose is the main product of photosynthesis and we rarely see the molecule’s L-form in nature.
Functional Groups
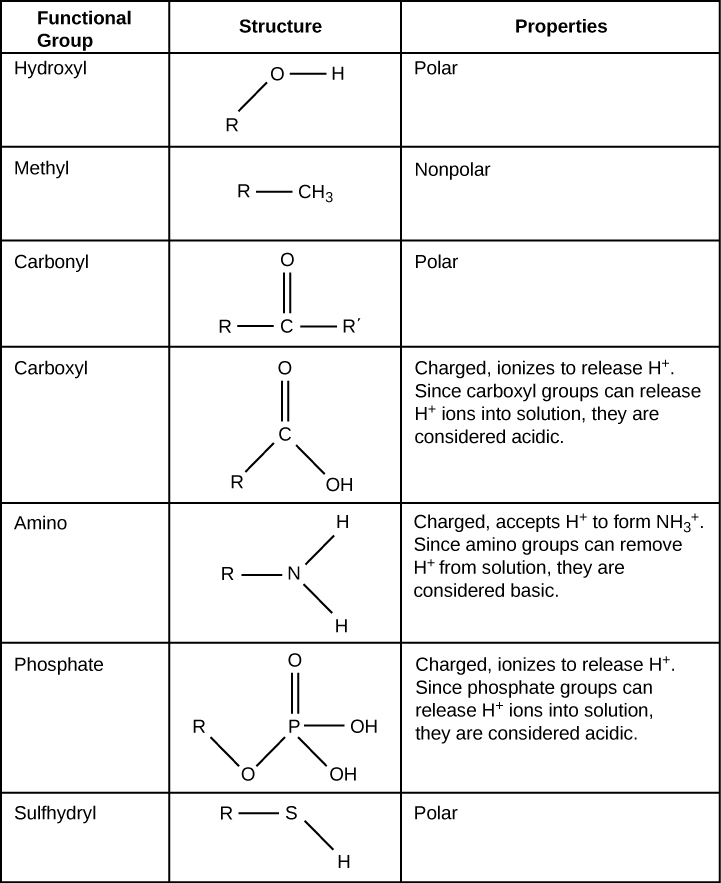
Functional groups are groups of atoms that occur within molecules and confer specific chemical properties to those molecules. We find them along the carbon backbone of macromolecules. Chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen form this carbon backbone. Molecules with other elements in their carbon backbone are substituted hydrocarbons.
The functional groups in a macromolecule are usually attached to the carbon backbone at one or several different places along its chain and/or ring structure. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
A functional group can participate in specific chemical reactions. Figure 2.27 shows some of the important functional groups in biological molecules. They include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl. These groups play an important role in forming molecules like DNA, proteins, carbohydrates, and lipids. We usually classify functional groups as hydrophobic or hydrophilic depending on their charge or polarity characteristics. An example of a hydrophobic group is the nonpolar methyl group. Among the hydrophilic functional groups is the carboxyl group in amino acids, some amino acid side chains, and the fatty acids that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the -COOH group resulting in the negatively charged -COO– group. This contributes to the hydrophilic nature of whatever molecule on which it is found. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly into and maintain the appropriate shape for functioning. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing (Figure 2.28) and the binding of an enzyme to its substrate.

Section Summary
The unique properties of carbon make it a central part of biological molecules. Carbon binds to oxygen, hydrogen, and nitrogen covalently to form the many molecules important for cellular function. Carbon has four electrons in its outermost shell and can form four bonds. Carbon and hydrogen can form hydrocarbon chains or rings. Functional groups are groups of atoms that confer specific properties to hydrocarbon (or substituted hydrocarbon) chains or rings that define their overall chemical characteristics and function.
Exercises
Glossary
aliphatic hydrocarbon: hydrocarbon consisting of a linear chain of carbon atoms
aromatic hydrocarbon: hydrocarbon consisting of closed rings of carbon atoms
enantiomers: molecules that share overall structure and bonding patterns, but differ in how the atoms are three dimensionally placed such that they are mirror images of each other
functional group: group of atoms on a carbon skeleton that confers specific chemical properties and reactivity
geometric isomer: isomer with similar bonding patterns differing in the placement of atoms alongside a double covalent bond
hydrocarbon: molecule that consists only of carbon and hydrogen
isomers: molecules that differ from one another even though they share the same chemical formula
organic molecule: any molecule containing carbon (except carbon dioxide)
structural isomers: molecules that share a chemical formula but differ in the placement of their chemical bonds
substituted hydrocarbon: hydrocarbon chain or ring containing an atom of another element in place of one of the backbone carbons
Media Attribution
- Figure 2.26 by Rao, A., Hawkins, A., Fletcher, S. and Ryan K. Department of Biology, Texas A&M University

