5.5 Oxidative Phosphorylation
Learning Objectives
By the end of this section, you will be able to:
- Describe the location of oxidative phosphorylation in the cell
- Describe the products of oxidative phosphorylation
The Mitochondrial Electron Transport Chain
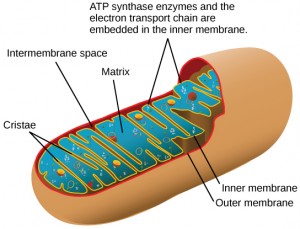
You have just read about two pathways in glucose catabolism—glycolysis and the citric acid cycle—that generate ATP. Most of the ATP generated during the aerobic catabolism of glucose, however, is not generated directly from these pathways. Rather, it derives from a process that begins with passing electrons through a series of chemical reactions to a final electron acceptor, oxygen. These reactions take place in specialized protein complexes located in the inner membrane of the mitochondria (Figure 5.17) of eukaryotic organisms and on the inner part of the cell membrane of prokaryotic organisms. The energy of the electrons is harvested and used to generate a electrochemical gradient across the inner mitochondrial membrane. The potential energy of this gradient is used to generate ATP. The entirety of this process is called oxidative phosphorylation.
The electron transport chain is the last component of aerobic respiration and is the only part of metabolism that uses atmospheric oxygen. There are four complexes composed of proteins, labeled I through IV in Figure 5.18a, and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes and in the plasma membrane of prokaryotes.
Electron transport is a series of chemical reactions that resembles a bucket brigade in that electrons from NADH and FADH2 are passed rapidly from one component to the next. In the fourth protein complex, the electrons are accepted by oxygen, the terminal acceptor. The oxygen with its extra electrons then combines with two hydrogen ions to form water. If there were no oxygen present in the mitochondrion, the electrons could not be removed from the system, and the entire electron transport chain would back up and stop. The mitochondria would be unable to generate new ATP in this way, and the cell would ultimately die from lack of energy. This is the reason we must breathe to draw in oxygen. Oxygen continuously diffuses into plants for this purpose. In animals, oxygen enters the body through the respiratory system.
In each transfer of an electron through the electron transport chain, the electron loses energy, but with some transfers, the energy is stored as potential energy by using it to pump hydrogen ions across the inner mitochondrial membrane into the intermembrane space, creating an electrochemical gradient owing to the H+ ions’ positive charge and their higher concentration on one side of the membrane.
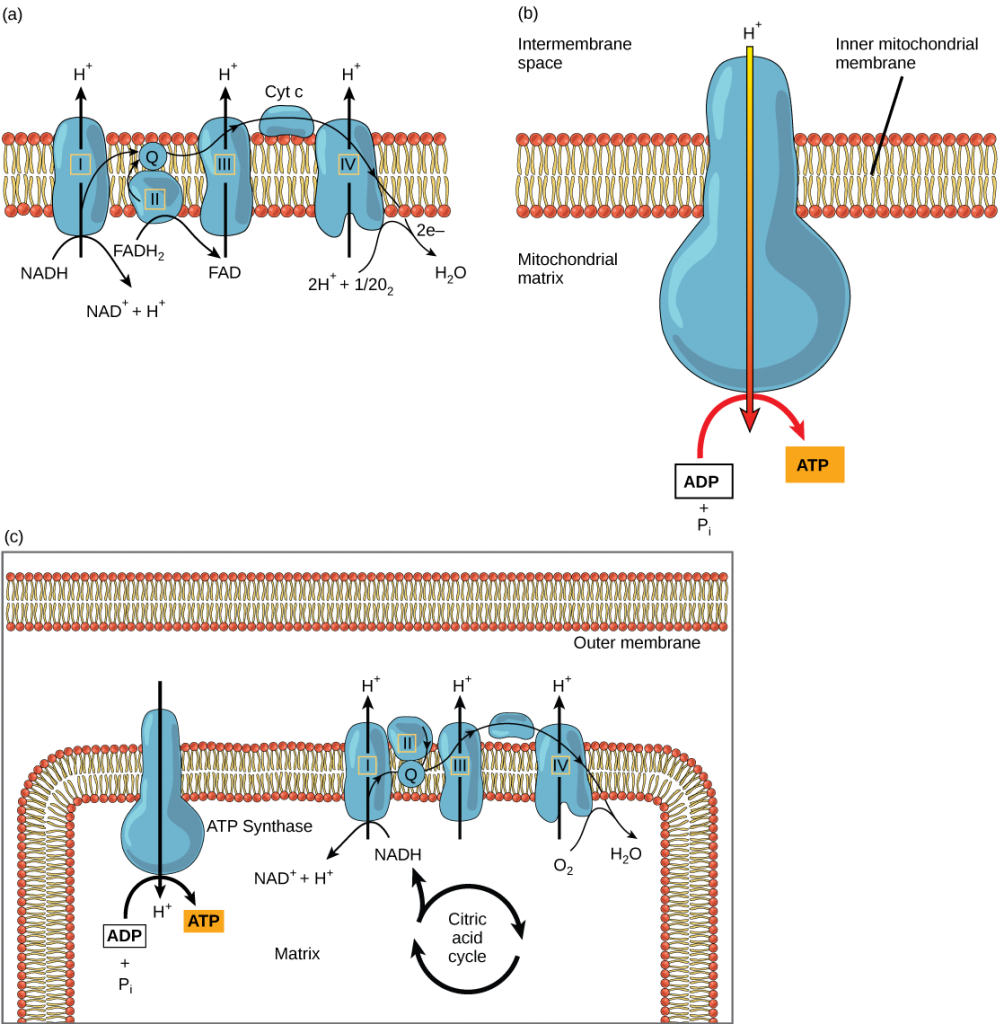
ATP Synthesis
Hydrogen ions diffuse through the inner membrane through an integral membrane protein called ATP synthase (Figure 5.18b). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient from the intermembrane space, where there are many mutually repelling hydrogen ions to the matrix, where there are few. The turning of the parts of this molecular machine regenerate ATP from ADP. This flow of hydrogen ions across the membrane through ATP synthase is called chemiosmosis (Figure 5.18c). Chemiosmosis is used to generate 90 percent of the ATP made during aerobic glucose catabolism. The electron transport chain and the production of ATP through chemiosmosis are collectively called oxidative phosphorylation.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the mitochondrial membrane. The NADH generated from glycolysis cannot easily enter mitochondria. Thus, electrons are picked up on the inside of the mitochondria by either NAD+ or FAD. Fewer ATP molecules are generated when FAD acts as a carrier. NAD+ is used as the electron transporter in the liver and FAD in the brain, so ATP yield depends on the tissue being considered.
Another factor that affects the yield of ATP molecules generated from glucose is that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Other molecules that would otherwise be used to harvest energy in glycolysis or the citric acid cycle may be removed to form nucleic acids, amino acids, lipids, or other compounds. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose.
CAREER CONNECTION
Mitochondrial Diseases
What happens when the critical reactions of cellular respiration do not proceed correctly? Mitochondrial diseases are genetic disorders of metabolism. Mitochondrial disorders can arise from mutations in nuclear or mitochondrial DNA, and they result in the production of less energy than is normal in body cells. Symptoms of mitochondrial diseases can include muscle weakness, lack of coordination, stroke-like episodes, and loss of vision and hearing. Most affected people are diagnosed in childhood, although there are some adult-onset diseases. Identifying and treating mitochondrial disorders is a specialized medical field. The educational preparation for this profession requires a college education, followed by medical school with a specialization in medical genetics. Medical geneticists can be board certified by the American Board of Medical Genetics and go on to become associated with professional organizations devoted to the study of mitochondrial disease, such as the Mitochondrial Medicine Society and the Society for Inherited Metabolic Disease.
Section Summary
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor for electrons removed from the intermediate compounds in glucose catabolism. The electrons are passed through a series of chemical reactions, with a small amount of free energy used at three points to transport hydrogen ions across the membrane. This contributes to the gradient used in chemiosmosis. As the electrons are passed from NADH or FADH2 down the electron transport chain, they lose energy. The products of the electron transport chain are water and ATP.
Exercises
Glossary
acetyl CoA: the combination of an acetyl group derived from pyruvic acid and coenzyme A which is made from pantothenic acid (a B-group vitamin)
ATP synthase: a membrane-embedded protein complex that regenerates ATP from ADP with energy from protons diffusing through it
chemiosmosis: the movement of hydrogen ions down their electrochemical gradient across a membrane through ATP synthase to generate ATP
citric acid cycle: a series of enzyme-catalyzed chemical reactions of central importance in all living cells that harvests the energy in carbon-carbon bonds of sugar molecules to generate ATP; the citric acid cycle is an aerobic metabolic pathway because it requires oxygen in later reactions to proceed
electron transport chain: a series of four large, multi-protein complexes embedded in the inner mitochondrial membrane that accepts electrons from donor compounds and harvests energy from a series of chemical reactions to generate a hydrogen ion gradient across the membrane
oxidative phosphorylation: the production of ATP by the transfer of electrons down the electron transport chain to create a proton gradient that is used by ATP synthase to add phosphate groups to ADP molecules
Media Attributions
- Figure 5.17 modification of work by Mariana Ruiz Villareal
- Figure 5.18(b) modification of work by Klaus Hoffmeier

