10.1 Mitosis
Learning Objectives
By the end of this section, you will be able to:
- Describe the three stages of interphase
- Discuss the behaviour of chromosomes during karyokinesis/mitosis and how the cytoplasmic content divides during cytokinesis
- Define the quiescent G0 phase
- Explain how the three internal control checkpoints occur at the end of G1, at the G2–M transition, and during metaphase
- Understand how the cell cycle is controlled by mechanisms that are both internal and external to the cell
- Describe the molecules that control the cell cycle through positive and negative regulation
The cell cycle is an ordered series of events involving cell growth and cell division that produces two new daughter cells. Cells on the path to cell division proceed through a series of precisely timed and carefully regulated stages of growth, DNA replication, and division that produce two genetically identical cells. The cell cycle has two major phases: interphase and the mitotic phase (Figure 10.1). During interphase, the cell grows and DNA is replicated. During the mitotic phase, the replicated DNA and cytoplasmic contents are separated and the cell divides, and the cell cytoplasm is typically partitioned by a third process of the cell cycle called cytokinesis. We should note, however, that interphase and mitosis (kayrokinesis) may take place without cytokinesis, in which case cells with multiple nuclei (multinucleate cells) are produced.
Watch this video about the cell cycle: https://www.youtube.com/watch?v=Wy3N5NCZBHQ
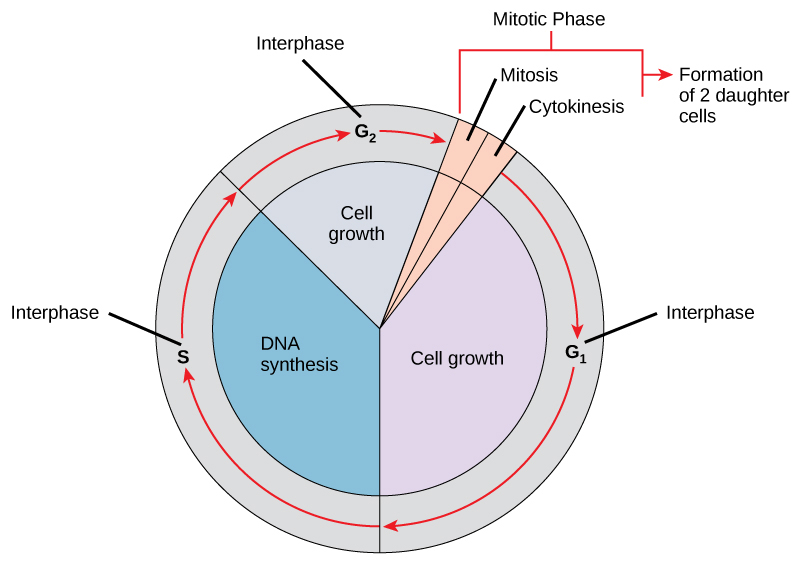
Interphase
During interphase, the cell undergoes normal processes while also preparing for cell division. For a cell to move from interphase to the mitotic phase, many internal and external conditions must be met. The three stages of interphase are called G1, S, and G2.
G1 Phase
The first stage of interphase is called the G1 phase, or first gap, because little change is visible. However, during the G1 stage, the cell is quite active at the biochemical level. The cell is accumulating the building blocks of chromosomal DNA and the associated proteins, as well as accumulating enough energy reserves to complete the task of replicating each chromosome in the nucleus.
S Phase
Throughout interphase, nuclear DNA remains in a semi-condensed chromatin configuration. In the S phase (synthesis phase), DNA replication results in the formation of two identical copies of each chromosome—sister chromatids—that are firmly attached at the centromere region. At this stage, each chromosome is made of two sister chromatids and is a duplicated chromosome. The centrosome is duplicated during the S phase. The two centrosomes will give rise to the mitotic spindle, the apparatus that orchestrates the movement of chromosomes during mitosis. The centrosome consists of a pair of rod-like centrioles at right angles to each other. Centrioles help organize cell division. Centrioles are not present in the centrosomes of many eukaryotic species, such as plants and most fungi.
G2 Phase
In the G2 phase, or second gap, the cell replenishes its energy stores and synthesizes the proteins necessary for chromosome manipulation. Some cell organelles are duplicated, and the cytoskeleton is dismantled to provide resources for the mitotic spindle. There may be additional cell growth during G2. The final preparations for the mitotic phase must be completed before the cell is able to enter the first stage of mitosis.
The Mitotic Phase
To make two daughter cells, the contents of the nucleus and the cytoplasm must be divided. The mitotic phase is a multistep process during which the duplicated chromosomes are aligned, separated, and moved to opposite poles of the cell, and then the cell is divided into two new identical daughter cells. The first portion of the mitotic phase, mitosis, is composed of five stages, which accomplish karyokinesis, nuclear division. The second portion of the mitotic phase, called cytokinesis, is the physical separation of the cytoplasmic components into two daughter cells.
Mitosis
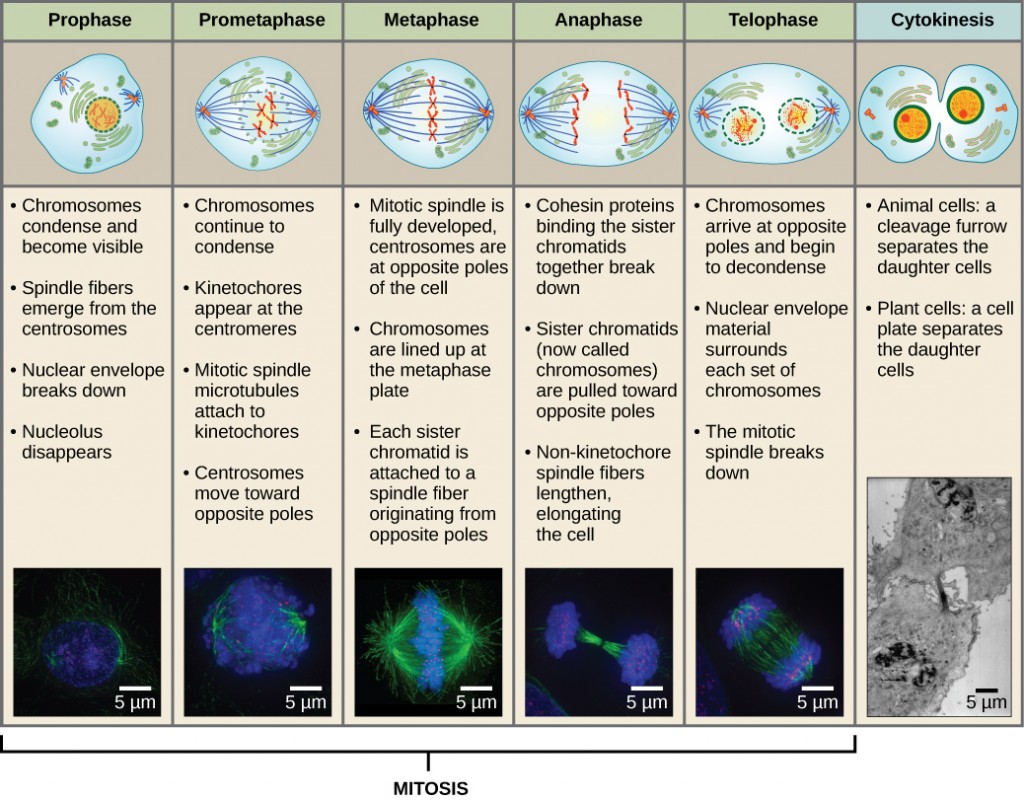
Mitosis is divided into a series of phases—prophase, prometaphase, metaphase, anaphase, and telophase—that result in the division of the cell nucleus (Figure 10.2).
Which of the following is the correct order of events in mitosis?
- Sister chromatids line up at the metaphase plate. The kinetochore becomes attached to the mitotic spindle. The nucleus re-forms and the cell divides. The sister chromatids separate.
- The kinetochore becomes attached to the mitotic spindle. The sister chromatids separate. Sister chromatids line up at the metaphase plate. The nucleus re-forms and the cell divides.
- The kinetochore becomes attached to metaphase plate. Sister chromatids line up at the metaphase plate. The kinetochore breaks down and the sister chromatids separate. The nucleus re-forms and the cell divides.
- The kinetochore becomes attached to the mitotic spindle. Sister chromatids line up at the metaphase plate. The kinetochore breaks apart and the sister chromatids separate. The nucleus re-forms and the cell divides.
During prophase, the “first phase,” several events must occur to provide access to the chromosomes in the nucleus. The nuclear envelope starts to break into small vesicles, and the Golgi apparatus and endoplasmic reticulum fragment and disperse to the periphery of the cell. The nucleolus disappears. The centrosomes begin to move to opposite poles of the cell. The microtubules that form the basis of the mitotic spindle extend between the centrosomes, pushing them farther apart as the microtubule fibres lengthen. The sister chromatids begin to coil more tightly and become visible under a light microscope.
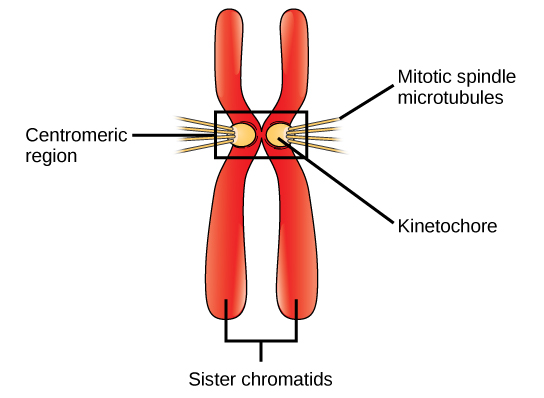
During prometaphase, many processes that were begun in prophase continue to advance and culminate in the formation of a connection between the chromosomes and cytoskeleton. The remnants of the nuclear envelope disappear. The mitotic spindle continues to develop as more microtubules assemble and stretch across the length of the former nuclear area. Chromosomes become more condensed and visually discrete. Each sister chromatid attaches to spindle microtubules at the centromere via a protein complex called the kinetochore (Figure 10.3). Once a mitotic fibre attaches to a chromosome, the chromosome will be oriented until the kinetochores of sister chromatids face the opposite poles. Eventually, all the sister chromatids will be attached via their kinetochores to microtubules from opposing poles. Spindle microtubules that do not engage the chromosomes are called polar microtubules. These microtubules overlap each other midway between the two poles and contribute to cell elongation. Astral microtubules are located near the poles, aid in spindle orientation, and are required for the regulation of mitosis.
During metaphase, all of the chromosomes are aligned in a plane called the metaphase plate, or the equatorial plane, midway between the two poles of the cell. The sister chromatids are still tightly attached to each other by cohesin proteins. At this time, the chromosomes are maximally condensed.
During anaphase, the sister chromatids at the equatorial plane are split apart at the centromere. Each chromatid, now called a chromosome, is pulled rapidly toward the centrosome to which its microtubule was attached. The cell becomes visibly elongated as the non-kinetochore microtubules slide against each other at the metaphase plate where they overlap.
During telophase, all of the events that set up the duplicated chromosomes for mitosis during the first three phases are reversed. The chromosomes reach the opposite poles and begin to decondense (unravel). The mitotic spindles are broken down into monomers that will be used to assemble cytoskeleton components for each daughter cell. Nuclear envelopes form around chromosomes.

This page of movies illustrates different aspects of mitosis. Watch the movie entitled “DIC microscopy of cell division in a newt lung cell” and identify the phases of mitosis.
LINK TO LEARNING
Revisit the stages of mitosis at this site.
Cytokinesis
Cytokinesis is the second part of the mitotic phase during which cell division is completed by the physical separation of the cytoplasmic components into two daughter cells. Although the stages of mitosis are similar for most eukaryotes, the process of cytokinesis is quite different for eukaryotes that have cell walls, such as plant cells.
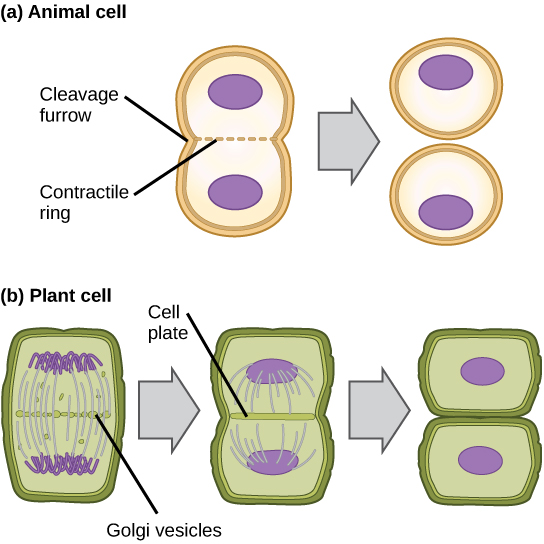
In cells such as animal cells that lack cell walls, cytokinesis begins following the onset of anaphase. A contractile ring composed of actin filaments forms just inside the plasma membrane at the former metaphase plate. The actin filaments pull the equator of the cell inward, forming a fissure. This fissure, or “crack,” is called the cleavage furrow. The furrow deepens as the actin ring contracts, and eventually the membrane and cell are cleaved in two (Figure 10.4a).
In plant cells, a cleavage furrow is not possible because of the rigid cell walls surrounding the plasma membrane. A new cell wall must form between the daughter cells. During interphase, the Golgi apparatus accumulates enzymes, structural proteins, and glucose molecules prior to breaking up into vesicles and dispersing throughout the dividing cell. During telophase, these Golgi vesicles are transported on microtubules to form a phragmoplast (a vesicular structure) at the metaphase plate. There, the vesicles fuse from the centre toward the cell walls; this structure is called a cell plate. As more vesicles fuse, the cell plate enlarges until it merges with the cell wall at the periphery of the cell. Enzymes use the glucose that has accumulated between the membrane layers to build a new cell wall of cellulose. The Golgi membranes become the plasma membrane on either side of the new cell wall (Figure 10.4b).
G0 Phase
Not all cells adhere to the classic cell-cycle pattern in which a newly formed daughter cell immediately enters interphase, closely followed by the mitotic phase. Cells in the G0 phase are not actively preparing to divide. The cell is in a quiescent (inactive) stage, having exited the cell cycle. Some cells enter G0 temporarily due to environmental conditions such as availability of nutrients, or stimulation by growth factors until an external signal triggers the onset of G1. Other cells that never or rarely divide, such as mature cardiac muscle and nerve cells, remain in G0 permanently (Figure 10.5).
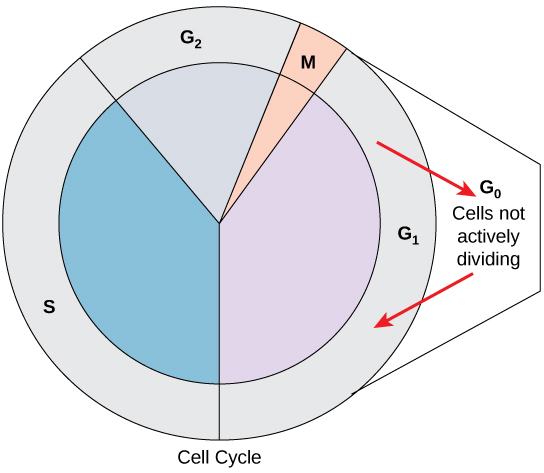
Control of the Cell Cycle
The length of the cell cycle is highly variable, even within the cells of a single organism. In humans, the frequency of cell turnover ranges from a few hours in early embryonic development, to an average of two to five days for epithelial cells, and to an entire human lifetime spent in G0 by specialized cells, such as cortical neurons or cardiac muscle cells.
There is also variation in the time that a cell spends in each phase of the cell cycle. When rapidly dividing mammalian cells are grown in a culture (outside the body under optimal growing conditions), the length of the cell cycle is about 24 hours. In rapidly dividing human cells with a 24-hour cell cycle, the G1 phase lasts approximately nine hours, the S phase lasts 10 hours, the G2 phase lasts about four and one-half hours, and the M phase lasts approximately one-half hour. By comparison, in fertilized eggs (and early embryos) of fruit flies, the cell cycle is completed in about eight minutes. This is because the nucleus of the fertilized egg divides many times by mitosis but does not go through cytokinesis until a multinucleate “zygote” has been produced, with many nuclei located along the periphery of the cell membrane, thereby shortening the time of the cell division cycle. The timing of events in the cell cycle of both “invertebrates” and “vertebrates” is controlled by mechanisms that are both internal and external to the cell.
Regulation of the Cell Cycle by External Events
Both the initiation and inhibition of cell division are triggered by events external to the cell when it is about to begin the replication process. An event may be as simple as the death of nearby cells or as sweeping as the release of growth-promoting hormones, such as human growth hormone (HGH or hGH). A lack of HGH can inhibit cell division, resulting in dwarfism, whereas too much HGH can result in gigantism. Crowding of cells can also inhibit cell division. In contrast, a factor that can initiate cell division is the size of the cell: As a cell grows, it becomes physiologically inefficient due to its decreasing surface-to-volume ratio. The solution to this problem is to divide.
Whatever the source of the message, the cell receives the signal, and a series of events within the cell allows it to proceed into interphase. Moving forward from this initiation point, every parameter required during each cell cycle phase must be met or the cycle cannot progress.
Regulation at Internal Checkpoints
It is essential that daughter cells be exact duplicates of the parent cell. Mistakes in the duplication or distribution of the chromosomes lead to mutations that may be passed forward to every new cell produced from the abnormal cell. To prevent a compromised cell from continuing to divide, there are internal control mechanisms that operate at three main cell cycle checkpoints at which the cell cycle can be stopped until conditions are favourable. These checkpoints occur near the end of G1, at the G2–M transition, and during metaphase (Figure 10.6).
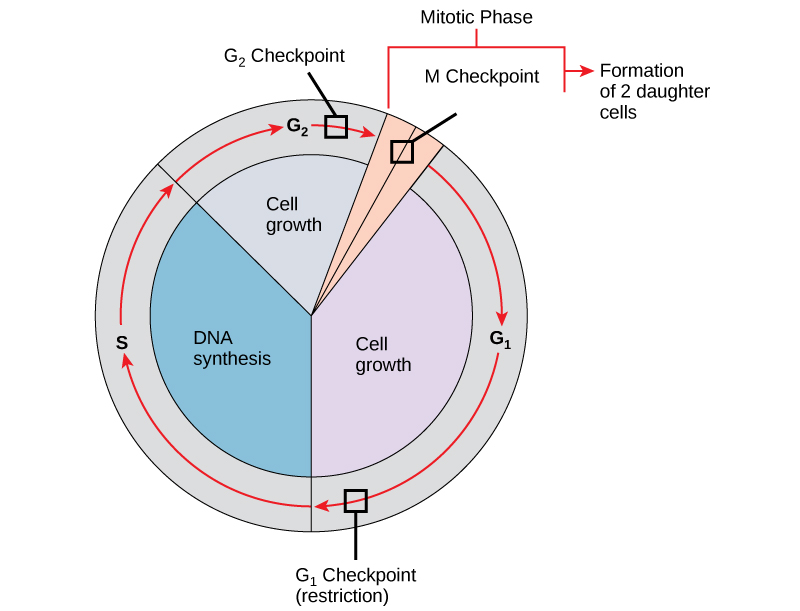
The G1 Checkpoint
The G1 checkpoint determines whether all conditions are favourable for cell division to proceed. The G1 checkpoint, also called the restriction point (in yeast), is a point at which the cell irreversibly commits to the cell division process. External influences, such as growth factors, play a large role in carrying the cell past the G1 checkpoint. In addition to adequate reserves and cell size, there is a check for genomic DNA damage at the G1 checkpoint. A cell that does not meet all the requirements will not be allowed to progress into the S phase. The cell can halt the cycle and attempt to remedy the problematic condition, or the cell can advance into G0 and await further signals when conditions improve.
The G2 Checkpoint
The G2 checkpoint bars entry into the mitotic phase if certain conditions are not met. As at the G1 checkpoint, cell size and protein reserves are assessed. However, the most important role of the G2 checkpoint is to ensure that all of the chromosomes have been replicated and that the replicated DNA is not damaged. If the checkpoint mechanisms detect problems with the DNA, the cell cycle is halted, and the cell attempts to either complete DNA replication or repair the damaged DNA.
The M Checkpoint
The M checkpoint occurs near the end of the metaphase stage of mitosis. The M checkpoint is also known as the spindle checkpoint because it determines if all the sister chromatids are correctly attached to the spindle microtubules. Because the separation of the sister chromatids during anaphase is an irreversible step, the cycle will not proceed until the kinetochores of each pair of sister chromatids are firmly anchored to spindle fibres arising from opposite poles of the cell.
Regulator Molecules of the Cell Cycle
In addition to the internally controlled checkpoints, there are two groups of intracellular molecules that regulate the cell cycle. These regulatory molecules either promote progress of the cell to the next phase (positive regulation) or halt the cycle (negative regulation). Regulator molecules may act individually, or they can influence the activity or production of other regulatory proteins. Therefore, the failure of a single regulator may have almost no effect on the cell cycle, especially if more than one mechanism controls the same event. However, the effect of a deficient or non-functioning regulator can be wide-ranging and possibly fatal to the cell if multiple processes are affected.
Positive Regulation of the Cell Cycle
Two groups of proteins, called cyclins and cyclin-dependent kinases (Cdks), are termed positive regulators. They are responsible for the progress of the cell through the various checkpoints. The levels of the four cyclin proteins fluctuate throughout the cell cycle in a predictable pattern (Figure 10.7). Increases in the concentration of cyclin proteins are triggered by both external and internal signals. After the cell moves to the next stage of the cell cycle, the cyclins that were active in the previous stage are degraded by cytoplasmic enzymes, as shown in Figure 10.7 below.
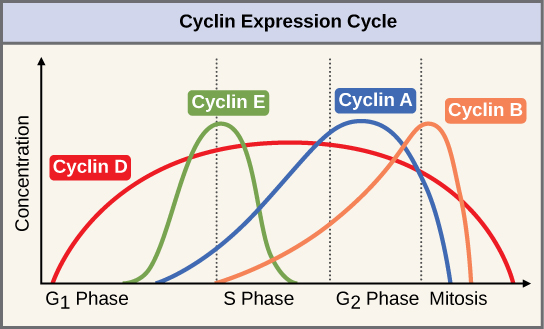
Cyclins regulate the cell cycle only when they are tightly bound to Cdks. To be fully active, the Cdk/cyclin complex must also be phosphorylated in specific locations to activate the complex. Like all kinases, Cdks are enzymes (kinases) that in turn phosphorylate other proteins. Phosphorylation activates the protein by changing its shape. The proteins phosphorylated by Cdks are involved in advancing the cell to the next phase (Figure 10.8). The levels of Cdk proteins are relatively stable throughout the cell cycle; however, the concentrations of cyclin fluctuate and determine when Cdk/cyclin complexes form. The different cyclins and Cdks bind at specific points in the cell cycle and thus regulate different checkpoints.
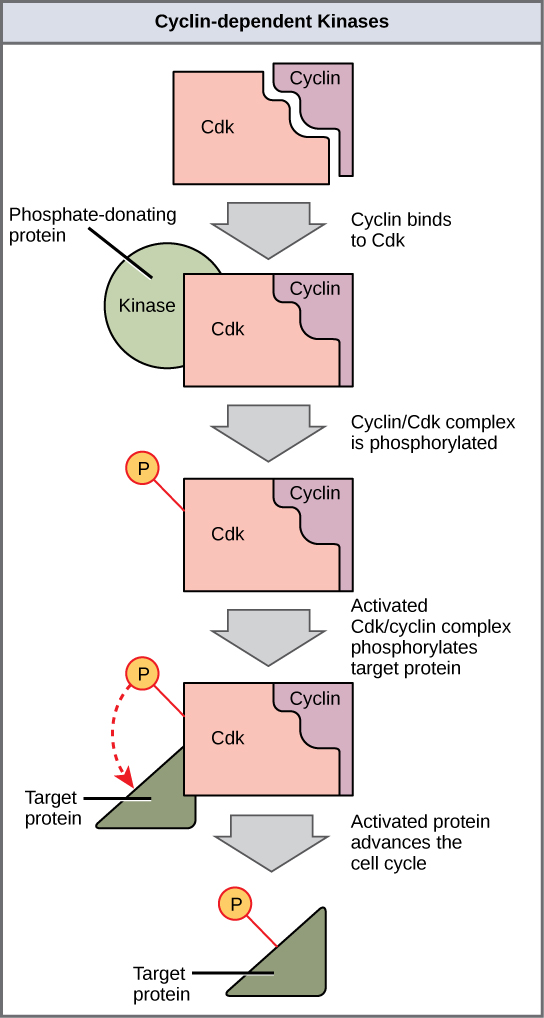
Because the cyclic fluctuations of cyclin levels are largely based on the timing of the cell cycle and not on specific events, regulation of the cell cycle usually occurs by either the Cdk molecules alone or the Cdk/cyclin complexes. Without a specific concentration of fully activated cyclin/Cdk complexes, the cell cycle cannot proceed through the checkpoints.
Although the cyclins are the main regulatory molecules that determine the forward momentum of the cell cycle, there are several other mechanisms that fine-tune the progress of the cycle with negative, rather than positive, effects. These mechanisms essentially block the progression of the cell cycle until problematic conditions are resolved. Molecules that prevent the full activation of Cdks are called Cdk inhibitors. Many of these inhibitor molecules directly or indirectly monitor a particular cell-cycle event. The block placed on Cdks by inhibitor molecules will not be removed until the specific event that the inhibitor monitors is completed.
Negative Regulation of the Cell Cycle
The second group of cell-cycle regulatory molecules are negative regulators, which stop the cell cycle. Remember that in positive regulation, active molecules cause the cycle to progress.
The best understood negative regulatory molecules are retinoblastoma protein (Rb), p53, and p21. Retinoblastoma proteins are a group of tumor-suppressor proteins common in many cells. We should note here that the 53 and 21 designations refer to the functional molecular masses of the proteins (p) in kilodaltons (a dalton is equal to an atomic mass unit, which is equal to one proton or one neutron or 1 g/mol). Much of what is known about cell-cycle regulation comes from research conducted with cells that have lost regulatory control. All three of these regulatory proteins were discovered to be damaged or non-functional in cells that had begun to replicate uncontrollably (i.e., became cancerous). In each case, the main cause of the unchecked progress through the cell cycle was a faulty copy of the regulatory protein.
Rb, p53, and p21 act primarily at the G1 checkpoint. p53 is a multi-functional protein that has a major impact on the commitment of a cell to division because it acts when there is damaged DNA in cells that are undergoing the preparatory processes during G1. If damaged DNA is detected, p53 halts the cell cycle and then recruits specific enzymes to repair the DNA. If the DNA cannot be repaired, p53 can trigger apoptosis, or cell suicide, to prevent the duplication of damaged chromosomes. As p53 levels rise, the production of p21 is triggered. p21 enforces the halt in the cycle dictated by p53 by binding to and inhibiting the activity of the Cdk/cyclin complexes. As a cell is exposed to more stress, higher levels of p53 and p21 accumulate, making it less likely that the cell will move into the S phase.
Rb, which largely monitors cell size, exerts its regulatory influence on other positive regulator proteins. In the active, dephosphorylated state, Rb binds to proteins called transcription factors, most commonly, E2F (Figure 10.9). Transcription factors “turn on” specific genes, allowing the production of proteins encoded by that gene. When Rb is bound to E2F, production of proteins necessary for the G1/S transition is blocked. As the cell increases in size, Rb is slowly phosphorylated until it becomes inactivated. Rb releases E2F, which can now turn on the gene that produces the transition protein, and this particular block is removed. For the cell to move past each of the checkpoints, all positive regulators must be “turned on,” and all negative regulators must be “turned off.”
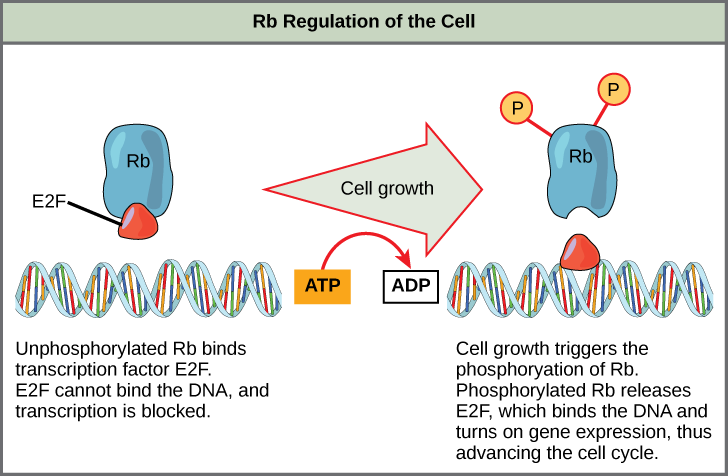
Rb and other proteins that negatively regulate the cell cycle are sometimes called tumor suppressors. Why do you think the name tumor suppressor might be appropriate for these proteins?
CONCEPT IN ACTION

Watch what occurs at the G1, G2, and M checkpoints by visiting this animation of the cell cycle.
Determine the Time Spent in Cell-Cycle Stages
Problem: How long does a cell spend in interphase compared to each stage of mitosis?
Background: A prepared microscope slide of whitefish blastula cross-sections will show cells arrested in various stages of the cell cycle. (Note: It is not visually possible to separate the stages of interphase from each other, but the mitotic stages are readily identifiable.) If 100 cells are examined, the number of cells in each identifiable cell-cycle stage will give an estimate of the time it takes for the cell to complete that stage.
Problem Statement: Given the events included in all of interphase and those that take place in each stage of mitosis, estimate the length of each stage based on a 24-hour cell cycle. Before proceeding, state your hypothesis.
Test your hypothesis: Test your hypothesis by doing the following:
- Place a fixed and stained microscope slide of whitefish blastula cross-sections under the scanning objective of a light microscope.
- Locate and focus on one of the sections using the low-power objective of your microscope. Notice that the section is a circle composed of dozens of closely packed individual cells.
- Switch to the medium-power objective and refocus. With this objective, individual cells are clearly visible, but the chromosomes will still be very small.
-
Switch to the high-power objective and slowly move the slide left to right, and up and down to view all the cells in the section (Figure 10.10). As you scan, you will notice that most of the cells are not undergoing mitosis but are in the interphase period of the cell cycle.
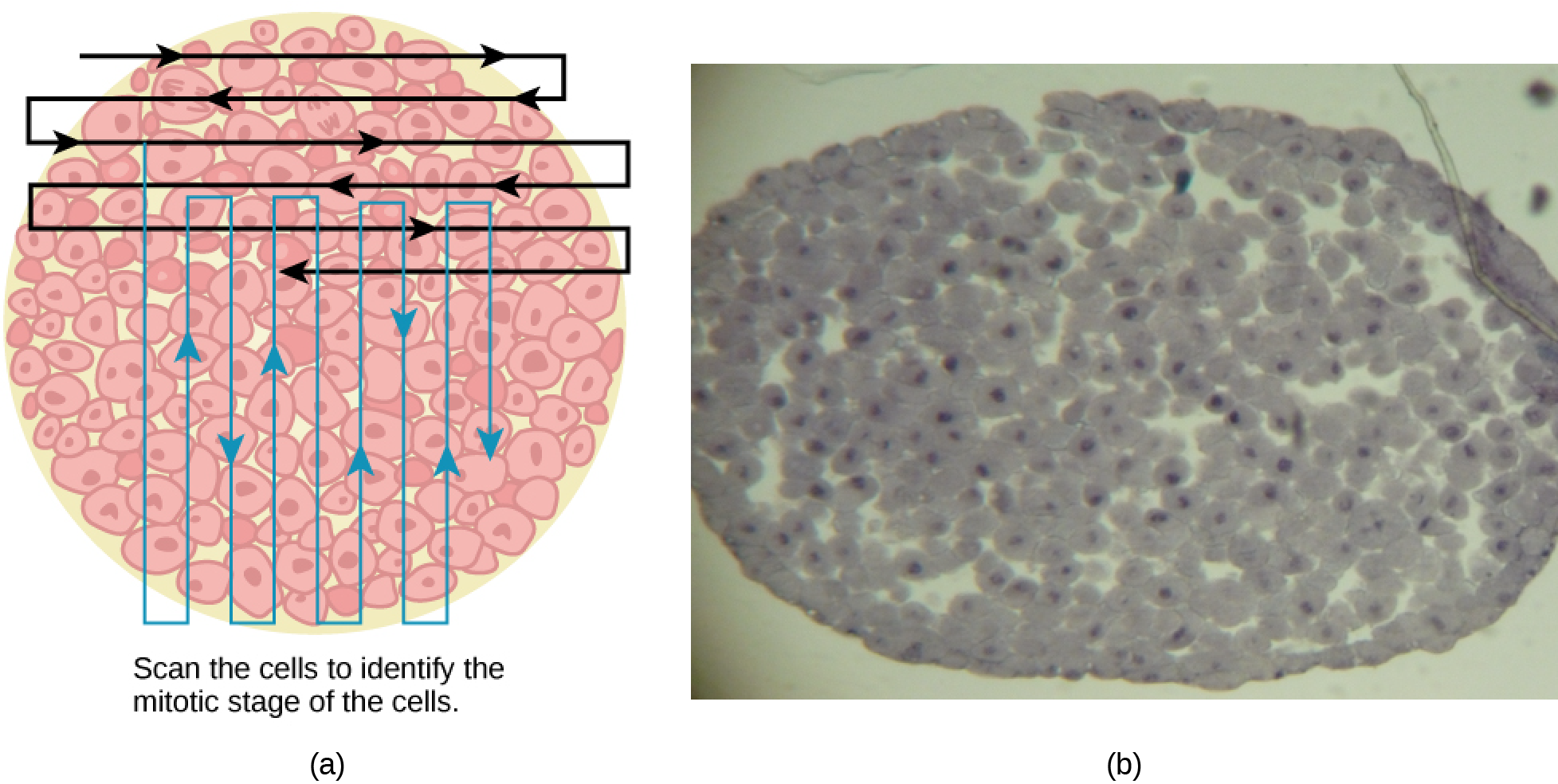
Figure 10.10 Slowly scan whitefish blastula cells with the high-power objective as illustrated in image (a) to identify their mitotic stage. (b) A microscopic image of the scanned cells is shown. (Credit “micrograph”: modification of work by Linda Flora; scale-bar data from Matt Russell) - Practice identifying the various stages of the cell cycle, using the drawings of the stages as a guide (Figure 10.10).
- Once you are confident about your identification, begin to record the stage of each cell you encounter as you scan left to right, and top to bottom across the blastula section.
- Keep a tally of your observations and stop when you reach 100 cells identified.
- The larger the sample size (total number of cells counted), the more accurate the results. If possible, gather and record group data prior to calculating percentages and making estimates.
Record your observations: Make a table similar to (Table 10.1) within which to record your observations.
| Table 10.1 Results of Cell Stage Identification | |||
|---|---|---|---|
| Phase or Stage | Individual Totals | Group Totals | Percent |
| Interphase | |||
| Prophase | |||
| Metaphase | |||
| Anaphase | |||
| Telophase | |||
| Cytokinesis | |||
| Totals | 100 | 100 | 100 percent |
Analyze your data/report your results: To find the length of time whitefish blastula cells spend in each stage, multiply the percent (recorded as a decimal) by 24 hours. Make a table similar to (Table 10.2) to illustrate your data.
| Table 10.2 Estimate of Cell Stage Length | ||
|---|---|---|
| Phase or Stage | Percent | Time in Hours |
| Interphase | ||
| Prophase | ||
| Metaphase | ||
| Anaphase | ||
| Telophase | ||
| Cytokinesis | ||
Draw a conclusion: Did your results support your estimated times? Were any of the outcomes unexpected? If so, discuss those events in that stage that may have contributed to the calculated time.
Section Summary
The cell cycle is an orderly sequence of events. Cells on the path to cell division proceed through a series of precisely timed and carefully regulated stages. In eukaryotes, the cell cycle consists of a long preparatory period, called interphase. Interphase is divided into G1, S, and G2 phases. Mitosis consists of five stages: prophase, prometaphase, metaphase, anaphase, and telophase. Mitosis is usually accompanied by cytokinesis, during which the cytoplasmic components of the daughter cells are separated either by an actin ring (animal cells) or by cell plate formation (plant cells).
Each step of the cell cycle is monitored by internal controls called checkpoints. There are three major checkpoints in the cell cycle: one near the end of G1, a second at the G2/M transition, and the third during metaphase. Positive regulator molecules allow the cell cycle to advance to the next stage of cell division. Negative regulator molecules monitor cellular conditions and can halt the cycle until specific requirements are met.
Exercises
Glossary
anaphase: the stage of mitosis during which sister chromatids are separated from each other
cell cycle: the ordered sequence of events that a cell passes through between one cell division and the next
cell cycle checkpoints: mechanisms that monitor the preparedness of a eukaryotic cell to advance through the various cell cycle stages
cell plate: a structure formed during plant-cell cytokinesis by Golgi vesicles fusing at the metaphase plate; will ultimately lead to formation of a cell wall to separate the two daughter cells
centriole: a paired rod-like structure constructed of microtubules at the centre of each animal cell centrosome
cleavage furrow: a constriction formed by the actin ring during animal-cell cytokinesis that leads to cytoplasmic division
cyclin: one of a group of proteins that act in conjunction with cyclin-dependent kinases to help regulate the cell cycle by phosphorylating key proteins; the concentrations of cyclins fluctuate throughout the cell cycle
cyclin-dependent kinase (Cdk): one of a group of protein kinases that helps to regulate the cell cycle when bound to cyclin; it functions to phosphorylate other proteins that are either activated or inactivated by phosphorylation
cytokinesis: the division of the cytoplasm following mitosis to form two daughter cells
G0 phase: a cell-cycle phase distinct from the G1 phase of interphase; a cell in G0 is not preparing to divide
G1 phase: (also, first gap) a cell-cycle phase; first phase of interphase centered on cell growth during mitosis
G2 phase: (also, second gap) a cell-cycle phase; third phase of interphase where the cell undergoes the final preparations for mitosis
interphase: the period of the cell cycle leading up to mitosis; includes G1, S, and G2 phases; the interim between two consecutive cell divisions
karyokinesis: mitotic nuclear division
kinetochore: a protein structure in the centromere of each sister chromatid that attracts and binds spindle microtubules during prometaphase
metaphase plate: the equatorial plane midway between two poles of a cell where the chromosomes align during metaphase
metaphase: the stage of mitosis during which chromosomes are lined up at the metaphase plate
mitosis: the period of the cell cycle at which the duplicated chromosomes are separated into identical nuclei; includes prophase, prometaphase, metaphase, anaphase, and telophase
mitotic phase: the period of the cell cycle when duplicated chromosomes are distributed into two nuclei and the cytoplasmic contents are divided; includes mitosis and cytokinesis
mitotic spindle: the microtubule apparatus that orchestrates the movement of chromosomes during mitosis
p21: cell-cycle regulatory protein that inhibits the cell cycle; its levels are controlled by p53
p53: cell-cycle regulatory protein that regulates cell growth and monitors DNA damage; it halts the progression of the cell cycle in cases of DNA damage and may induce apoptosis
prometaphase: the stage of mitosis during which mitotic spindle fibres attach to kinetochores
prophase: the stage of mitosis during which chromosomes condense and the mitotic spindle begins to form
quiescent: describes a cell that is performing normal cell functions and has not initiated preparations for cell division
retinoblastoma protein (Rb): regulatory molecule that exhibits negative effects on the cell cycle by interacting with a transcription factor (E2F)
S phase: the second, or synthesis phase, of interphase during which DNA replication occurs
telophase: the stage of mitosis during which chromosomes arrive at opposite poles, decondense, and are surrounded by new nuclear envelopes
Media Attributions
- Figure 10.2 “diagrams”: modification of work by Mariana Ruiz Villareal; “mitosis micrographs”: modification of work by Roy van Heesbeen; “cytokinesis micrograph”: modification of work by the Wadsworth Center, NY State Department of Health; donated to the Wikimedia foundation; scale-bar data from Matt Russell
- Figure 10.7 modification of work by WikiMiMa/Wikimedia Commons
- Figure 10.10 “micrograph”: modification of work by Linda Flora; scale-bar data from Matt Russell

